ANSYS MEDICAL DEVICE SIMULATION SOLUTIONS
Biomedical companies rely on Ansys software to keep us alive and deal with their unique industry challenges. The ability to innovate as well as provide the safe products that doctors and patients trust are crucial to medical device companies. In order to comply with regulatory constraints and avoid unnecessary clinical testing, companies turn to the trusted market leading analysis software Ansys.
With greater regulatory acceptance then ever before, computational modeling with Ansys allows companies to test in silico in order to reduce or avoid costly ‘build and test’ cycles. Parametric variation of device parameters and patient specific models can build confidence in a wide range of scenarios. The depth and breadth of the Ansys portfolio of software ensures you will be able to simulate your device’s true behavior in a physiologically real environment.
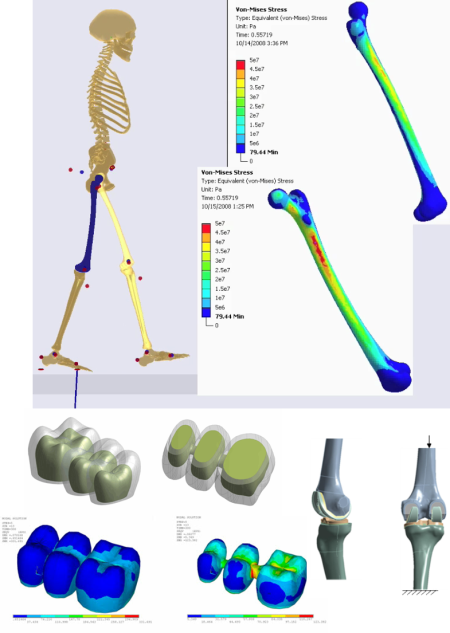
From fracture fixation all the way to full joint replacement, orthopedic implants are a key factor for quality of life for much of the population. Whereas the original bones and joints are constantly repaired and protected by the body, artificial implants are on their own and in the best case will be ignored by the immune system. With each patient being different and the stakes for failure being multiple surgical interventions, successful medical implant companies will use the Ansys portfolio of software to get the most out of their products:
- Patient specific modeling from scan data allows geometry and material properties to come directly from the patient.
- Nonlinear materials modeling capabilities including hyperelasticity, viscoelasticity, pore pressure coupled elements, shape memory alloys and more.
- Nonlinear contact modeling including full frictional as well as wear models.
- Use musculoskeletal modeling to generate physiological loads for a joint or bone.
- Use parametric analysis to optimize for performance and robustness.
- Fatigue calculations to simulate cycles to failure or wear.
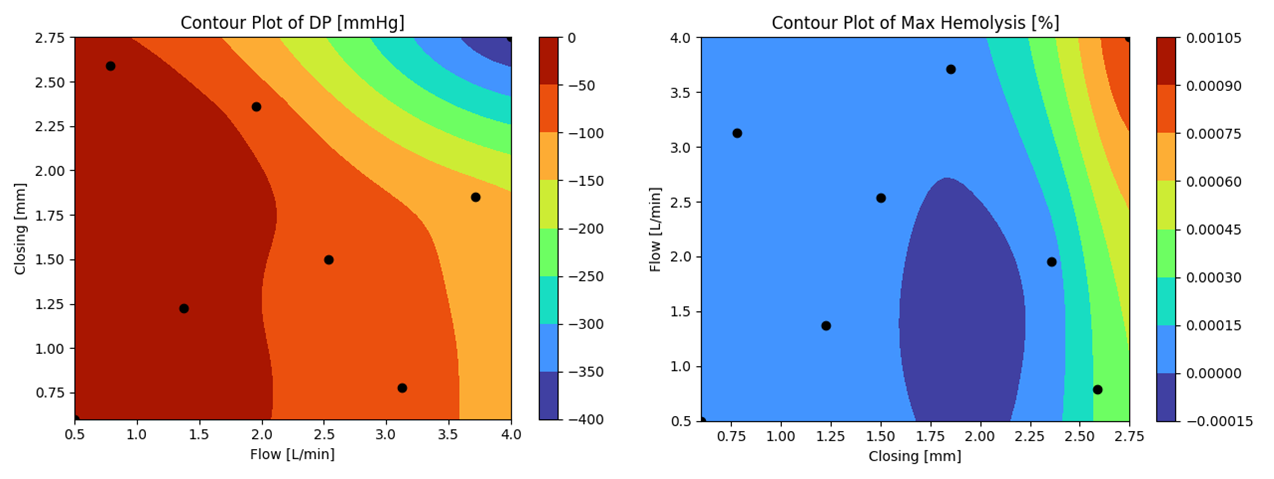
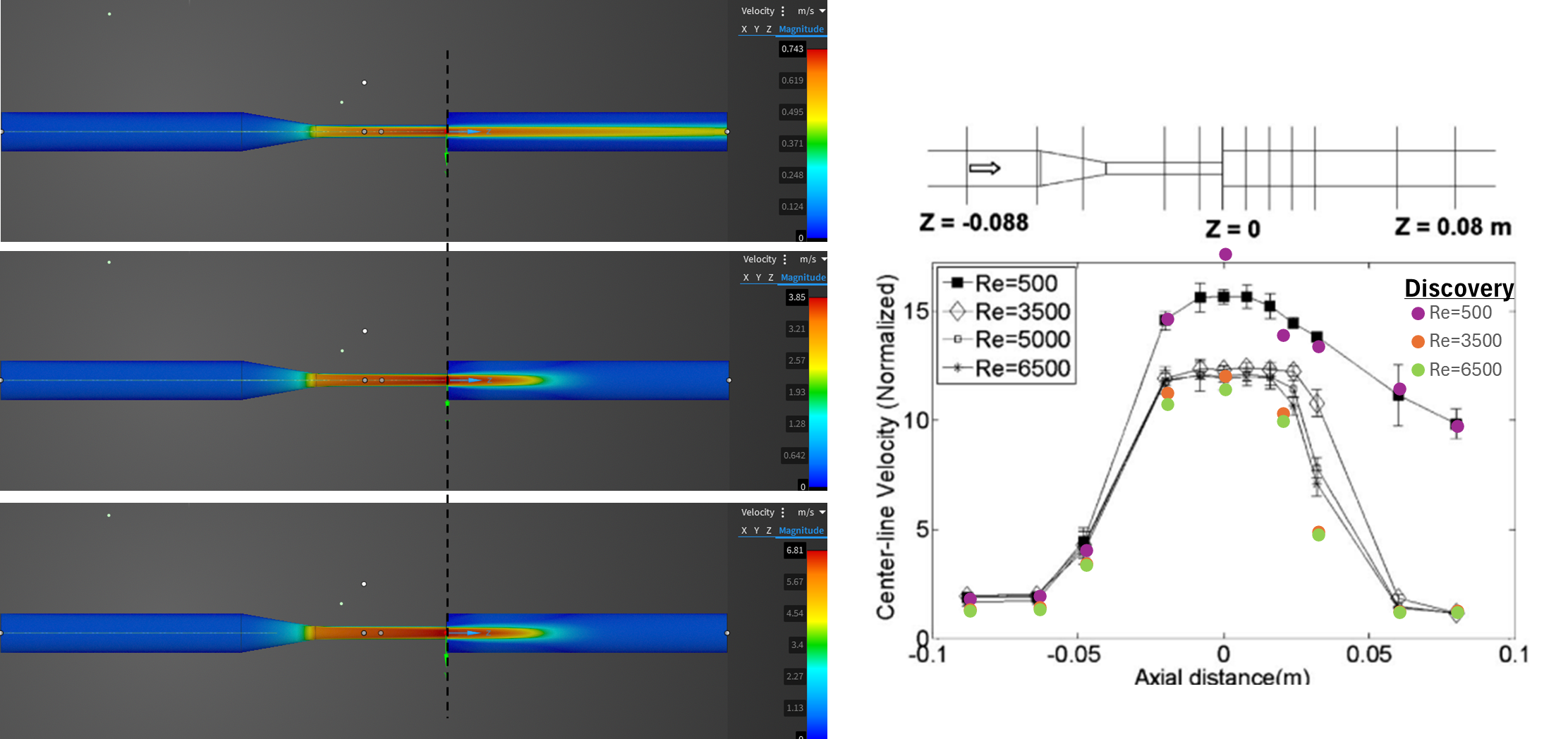
The circulatory system is one of the wonders of evolution, allowing us to become larger organisms by nourishing and oxygenating all 37 trillion cells in our body. Interventions in this engineering marvel require both a thorough understanding of the overall system and how a product will function within it. Ansys has the diversity of software to model the true behavior of stents, heart valves, heart pumps, endovascular devices, pacemakers and others.
- Model the cardiovascular system with fidelity with the most powerful and trusted CFD tools in the world.
- Fluid-Structure-Interaction (FSI) captures the effect of the flow on deformable blood vessels and vice versa.
- Nonlinear Shape Memory Alloys (SMA) and other material models capture the true behavior of devices.
- Systems modeling allows represent the full circulatory system outside of the CFD model through Reduced Order Modeling (ROM).
- Magnetohydrodynamic capabilities for modeling magnetic-drive pumps.
- Patient specific models of vessels can be created from scan data and used in the simulation.
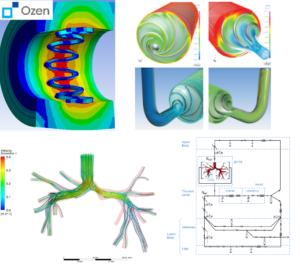
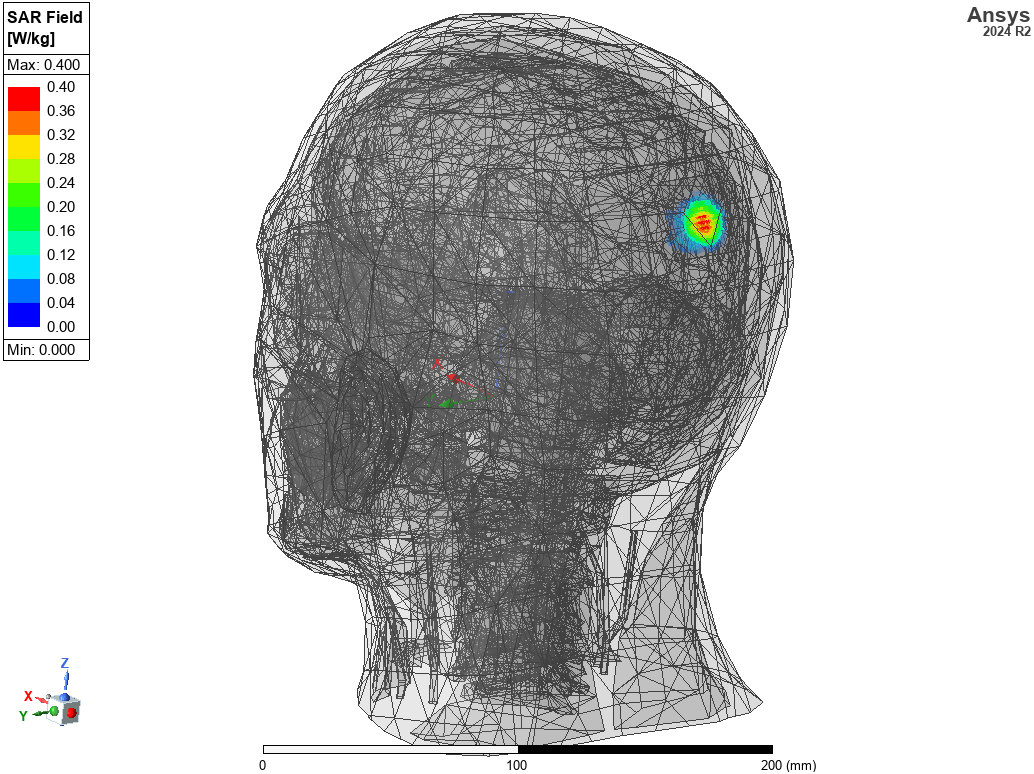
(clockwise from left) Electric field distribution around body from an implanted antenna, catheter flow simulation, magnetic field distribution in an MRI machine.
Safely diagnosing the human body gives care providers the information that they need to save lives. Advances in sensing and diagnostic technology also accelerate our understanding of the human body, leading to groundbreaking research and advances. In the delicate balance between sensing power and patient safety, Ansys is the difference.
- Design and simulate multiphysical sensing equipment with MEMS capabilities of Ansys.
- Increase sensitivity and reduce patient danger through smart antenna design and SAR evaluation on virtual humans.
- For catheters simulate the full use case with nonlinear materials and contacts to assure product quality.
- Simulate and try many different designs with inexpensive in silico parametric design analysis.
“[At FDA,] we’ve been looking at computational modeling as a fourth pillar of pre-market evaluation,” – Deputy Director for Science, FDA, Bill Maisel
Computational modeling tools like Ansys are becoming an ever more important piece of the regulatory approval process. The FDA has given their blessing to computational modeling in all stages of a product development cycle. Smart companies take advantage of simulation to demonstrate their product capabilities and even get design changes approved without additional clinical trials.
- Simulate with confidence by using the market leading FEA/CFD/Electromagnetic tools from Ansys.
- Try many variations in silico, get the most out of your design process.
- Reduce cost of physical testing by using simulation to gain more insight into your product’s behavior.
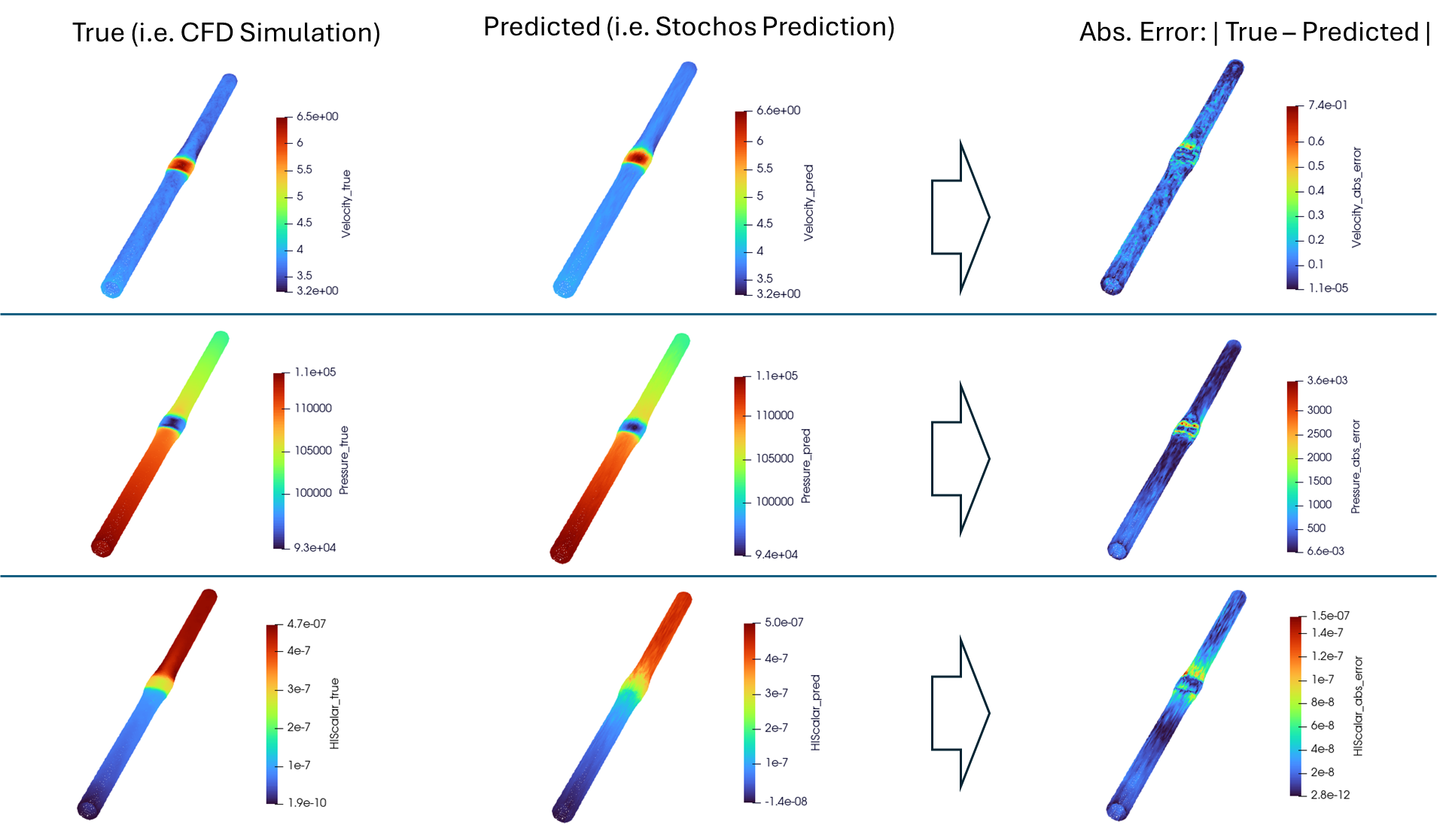
(top to bottom) Physical test of a 4 point bend and fixation scenario replicated in simulation, illustrations of the stochastic and robust design concepts, variations in femur and fixation plate geometry that can be tested in silico.